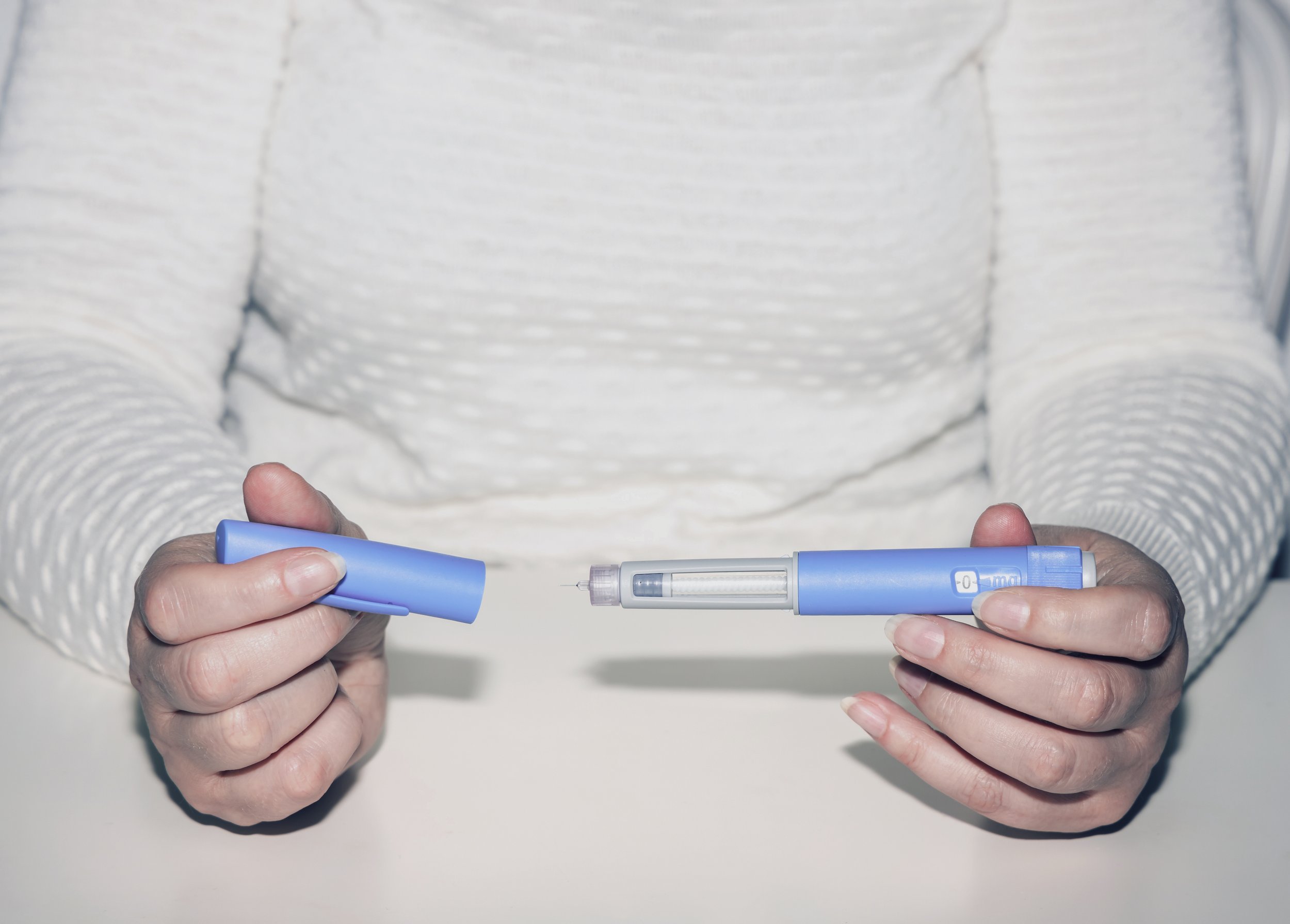
Clinical Need
Risk of Particles
Particle contamination is regarded by global regulatory agencies and pharmaceutical industry as an indicator of overall product quality and may impact patient safety and product efficacy.
Patients administered particles can develop serious to life-threatening immune reaction or respond poorly to therapy.
Biologic Particles and Glass Delamination (Particles) are an ongoing packaging challenge for drug delivery devices.
Biologic Particles
Stabilisation is the most important formulation challenge. Over 50% of biologic formulation projects experience delays of >12 months. Some 10% experience complete failure.
Glass Delamination (Particles)
Over 33% of particulate recalls have identified glass-derived particulate as the primary reason, approximately two recalls per year on average.
Problematic Coatings
Lubricative and barrier coatings have been used in drug delivery to enable desirable device performance and compatibility.
Yet, current coatings can cause adverse effects with various pharmaceutical drugs, alter immunologic response and ultimately affect patient safety.
Silicone Coatings and Fluoropolymer Coatings are an ongoing concern in injectable drug delivery.
Silicone Coatings
Silicone has a direct effect on the stability of 10–15% of the products in the global biologics pipeline.
Fluoropolymer Coatings
Both the EU and the US having existing and upcoming legislation aiming to mitigate, or even ban, the use of fluoropolymers.
